● Appling principle:
ICH Harmonised Tripartite Guideline Q1A_R2 “Stabiltiy Testing of New Drug Substances and Products”
● Long term work ability:
It can continuously and non-stoply work for 2 years and it can automatically defrost.
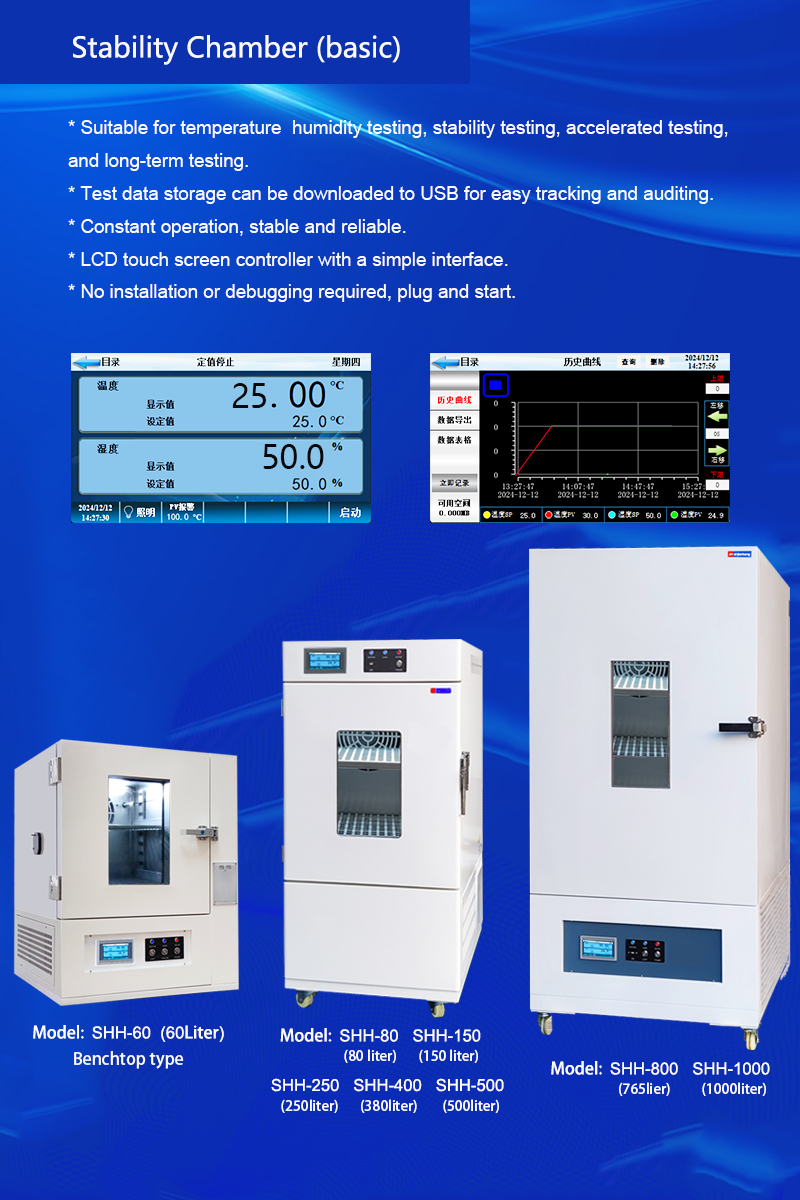
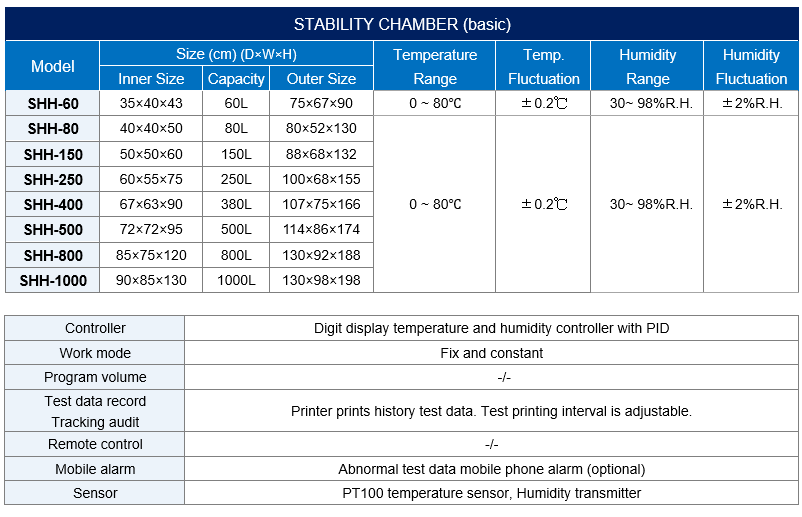
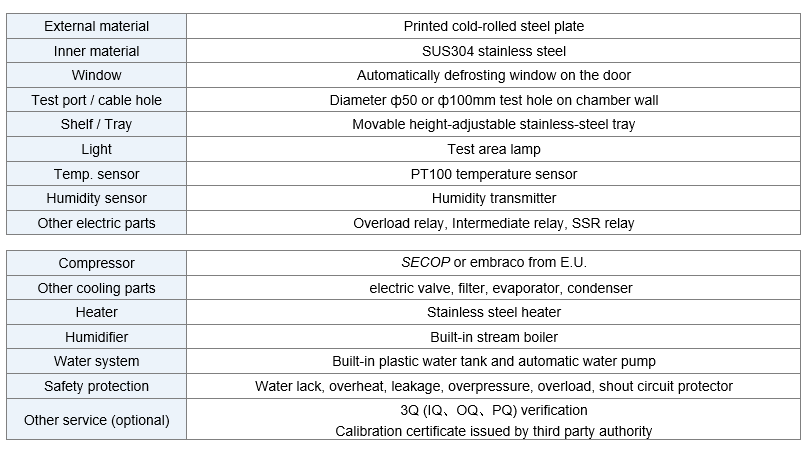

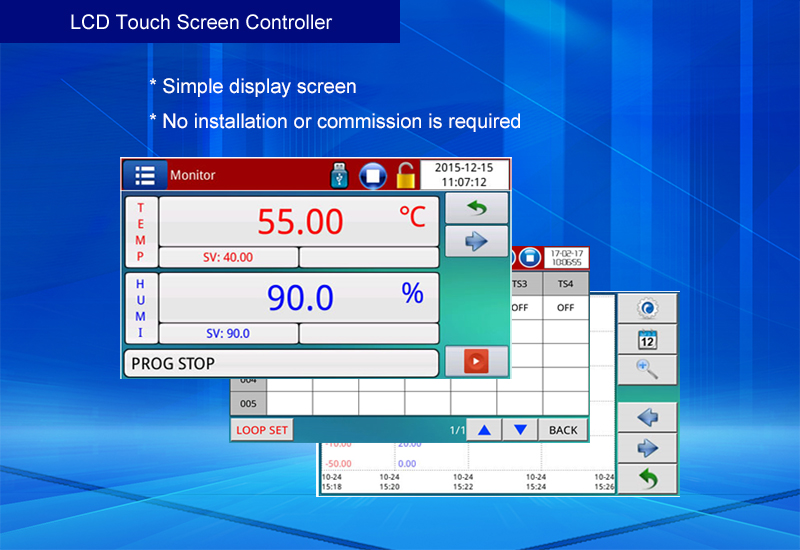
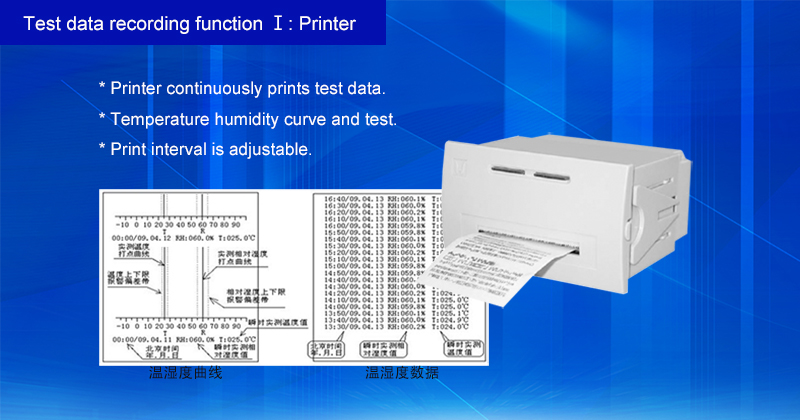
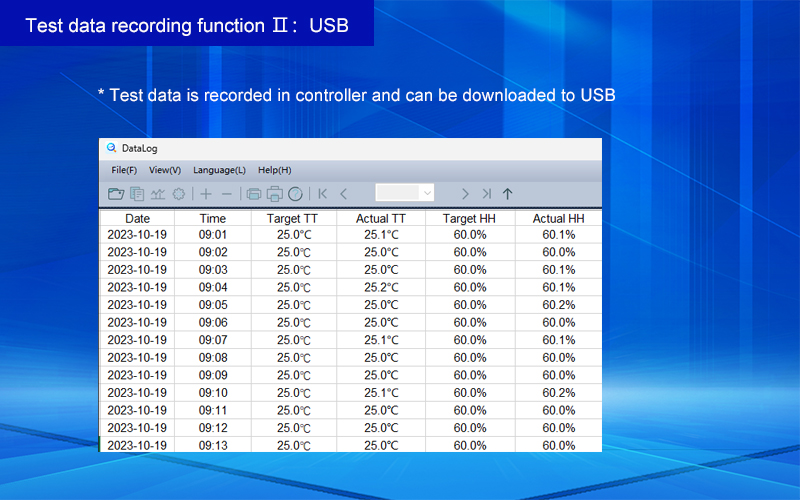


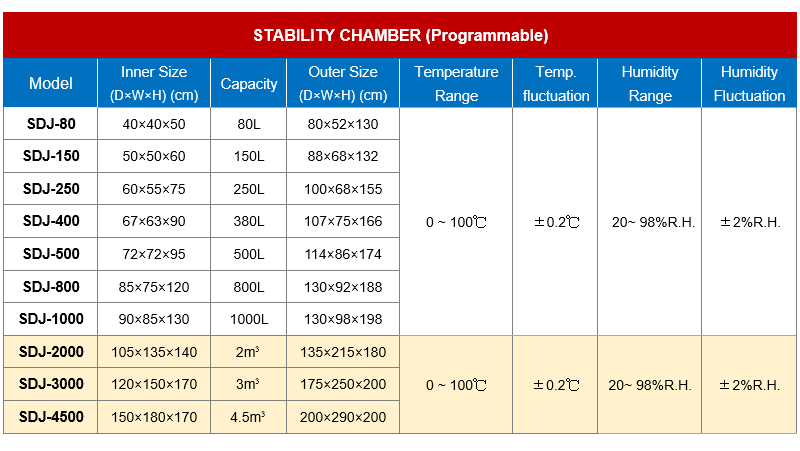

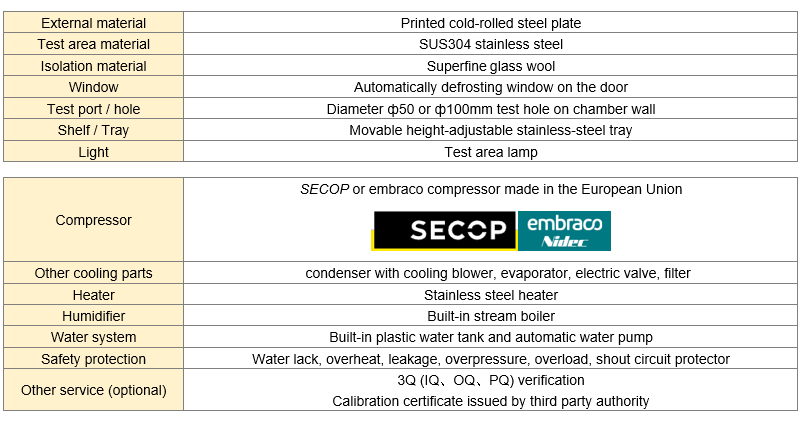
Model | Capacity | Work Mode | Test Data Record Method | Price (USD) |
SHH-60 | 60L | Constant | USB Download | 1200 |
SHH-80 | 80L | Constant | USB Download | 1250 |
SHH-150 | 150L | Constant | USB Download | 1550 |
SHH-250 | 250L | Constant | USB Download | 1750 |
SHH-400 | 380L | Constant | USB Download | 2300 |
SHH-500 | 500L | Constant | USB Download | 2600 |
SHH-800 | 765L | Constant | USB Download | 3300 |
SHH-1000 | 1000L | Constant | USB Download | 3600 |
SDJ-60 | 60L | Constant and Programmable | Printer and USB Download | 1450 |
SDJ-80 | 80L | Constant and Programmable | Printer and USB Download | 1500 |
SDJ-150 | 150L | Constant and Programmable | Printer and USB Download | 1750 |
SDJ-250 | 250L | Constant and Programmable | Printer and USB Download | 2000 |
SDJ-400 | 380L | Constant and Programmable | Printer and USB Download | 2550 |
SDJ-500 | 500L | Constant and Programmable | Printer and USB Download | 2900 |
SDJ-800 | 765L | Constant and Programmable | Printer and USB Download | 3500 |
SDJ-1000 | 1000L | Constant and Programmable | Printer and USB Download | 3800 |
SDJ-2000 | 2000L | Constant and Programmable | Printer and USB Download | 5400 |
SDJ-3000 | 3000L | Constant and Programmable | Printer and USB Download | 7500 |
SDJ-4500 | 4500L | Constant and Programmable | Printer and USB Download | 9600 |
* If you want also illumination function, please see the website of Photo Stability Chamber: https://www.shjianhengyiqi.com/product/503.html
* Please contact us for more technical information or quotation.
* If you want to specially customize the chamber, please contact us.
Sales email: shjianheng@gmail.com
WeChat: +8613816088813
.
Drug Stability Chamber: A Brief Introduction
A Drug Stability Chamber is specialized environmental equipment designed to evaluate pharmaceutical product shelf life under controlled conditions. These precision instruments simulate various temperature, humidity, and light exposure scenarios required by international regulatory guidelines (ICH, FDA, WHO).
Core PurposeThe chambers conduct long-term (25°C/60% RH), intermediate (30°C/65% RH), and accelerated stability (40°C/75% RH) testing to determine drug expiration dates, storage requirements, and degradation kinetics. Photostability variants test light sensitivity using defined UV and visible light sources per ICH Q1B guidelines.
Technical CapabilitiesAdvanced systems maintain temperature within ±0.5°C and relative humidity within ±3% through microprocessor-controlled sensors and forced air circulation. Key features include stainless steel construction, redundant safety systems, continuous data logging, and compliance with 21 CFR Part 11 for electronic records integrity.
Regulatory ImportanceThese chambers are essential for Good Manufacturing Practice (GMP) compliance and new drug applications. They enable pharmaceutical companies to demonstrate product quality, safety, and efficacy throughout the proposed shelf life, ensuring patient health protection while meeting stringent global regulatory standards for market authorization.
They serve quality control laboratories, R&D facilities, and contract research organizations (CROs) in supporting formulation development, packaging studies, and batch release testing